With growing momentum, stem cell therapies of all types are creating a huge buzz in the MS community. While one form of stem cell therapy – that intended to regenerate damaged nervous system tissue – is clearly still in its experimental infancy, another, hematopoietic stem cell therapy, which seeks to reboot the immune system, has been used on MS patients for almost 20 years. Early attempts at using HSCT to treat MS, though at times producing encouraging results, were fraught with danger, with as many as 10% of test subjects dying as a direct result of the procedure. Recent refinements in technique, better patient selection, and a growing knowledge base are now bringing HSCT closer to mainstream use as efficacy rates have soared and mortality rates have dropped dramatically. In the case of the best treatment centers, mortality rates have dropped below 1%, with no deaths reported over the last five or so years.
It’s very important to understand that there are currently two wholly separate and completely different approaches being explored for using stem cells to treat multiple sclerosis. Both hold tremendous promise but go about their business in entirely different ways, and care must be taken to never confuse the two. Regenerative stem cell therapies, almost all using some form of mesenchymal stem cells, seek to repair brain and spine tissues damaged by MS, while HSCT is focused entirely on the immune system and does not directly address damaged nervous system tissues at all. This post will deal exclusively with HSCT. For more info on the differences between these two stem cell methodologies, and more details on the experimental regenerative therapies, please refer to a previous overview of stem cell therapies for MS that appeared on Wheelchair Kamikaze last fall (click here).
So, first things first – what exactly is HSCT? In practice, HSCT is very similar to the bone marrow transplants that have been used to treat patients with leukemia and other cancers of the blood for decades. As a therapy for multiple sclerosis, the process begins by collecting a patient’s own stem cells, either through bone marrow harvesting or blood draws. Once collected, these stem cells are stored, and sometimes multiplied, in sterile laboratory conditions.
Then comes the dramatic part – the patient’s immune system is ablated (a polite way of saying destroyed) over the course of several days using powerful chemotherapy agents. Depending on the clinic treating the patient, a variety of drugs or combination of drugs is used, some more intense than others. The goal of this “conditioning regimen” is to leave the subject with no functioning immune system, obviously a very vulnerable state during which the patient must be kept in isolation to prevent exposure to any possible contaminants or infectious agents. While undergoing this conditioning regimen, patients typically suffer many of the common side effects of chemotherapy, including hair loss and nausea.
Once the immune system has been eradicated, the previously harvested stem cells are intravenously infused back into the patient’s body, where over the course of several weeks they rebuild the immune system, effectively giving the patient an entirely new array of immune cells. In theory, this brand-new immune system shouldn’t have the destructive tendencies that led their old immune cells to attack the patient’s own central nervous system tissues, the mechanism that is believed to cause the damage and lesions that give multiple sclerosis its name.
Though this may seem like a sledgehammer approach to treating MS, recent studies have shown HSCT to be astoundingly effective when used on properly selected patients. One recently published study followed 52 Swedish MS patients that were treated with HSCT (click here). At five years, relapse free survival was 87%, MRI event free survival 85%, EDSS score progression free survival 77%, and disease-free survival (no relapses, no new MRI lesions, and no EDSS progression) was 68%. The presence of Gadolinium enhancing lesions prior to HSCT was associated with a significantly higher degree of favorable outcome (79% exhibiting disease-free survival at five years). The study’s authors conclude that that “HSCT is a very effective treatment of inflammatory active MS and can be performed with a high degree of safety at experienced centers.” Other recent studies looking at the efficacy of HSCT have found similar results (click here and here). Outcomes such as these give ample reason to sit up and take notice, despite the admittedly frightening prospect of the use of intense chemotherapy conditioning regimens.
One must keep in mind that despite recent advances in treating MS with a new generation of immunosuppressant drugs, the disease in its most serious forms remains a brutal beast intent on laying waste to many of those it afflicts. Next generation MS drugs such as Tysabri, Gilenya, and Tecfidera are proving to be potent in managing the disease in those patients on whom they are effective, but these drugs must be taken indefinitely and each has their own set of possible serious side effects that give many patients pause. HSCT, on the other hand, is meant to be a one-time treatment, after which properly selected and treated patients are proving to show a remarkable degree of sustained disease-free existence, with some even experiencing a reversal in disability status (click here). While no responsible person is calling HSCT a cure for MS, and undergoing the treatment is no walk in the park (intense chemotherapy is serious business), years of disease-free life without the indefinite use of drugs is the stuff of most MSer’s dreams.
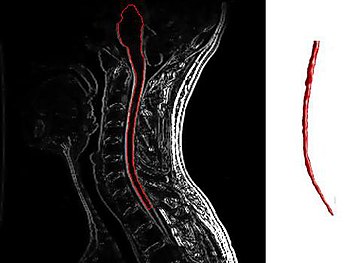
HSCT has been proven to work best on patients with very active inflammatory disease (those “properly selected patients” I keep talking about), meaning patients whose disease is marked by frequent relapses and enhancing lesions on their MRI images. Many if not most RRMS patients fall into this category, but unfortunately far fewer SPMS and PPMS patients fit the bill. Study after study (click here and here) has shown that the most important criteria for identifying patients on whom HSCT has the best chance of success is the presence of enhancing lesions as detected by MRI.
Enhancing lesions are a sign that the immune system is actively causing inflammation within a patient’s central nervous system, while the lack of enhancing lesions signals that the disease is being driven by some other, as yet undiscovered, mechanism. A comprehensive overview of HSCT results on MS patients worldwide conducted by Brazilian researchers came up with the following recommendations for selecting patients who might benefit from HSCT: “… the forms of the disease that might benefit from transplantation are: relapsing remitting, primary or secondary progressive, and the “malignant” form, provided there is evidence of inflammatory activity at the time of transplant indication.” These researchers further suggest that the treatment not be given to patients who have lost the ability to walk, with the exception of those suffering from extremely aggressive MS who have accumulated disability quickly. It’s also very important that patients be relatively healthy aside from their MS, hearty enough to withstand the rigors of a short burst of intense chemotherapy.
Unfortunately, as is illustrated by the above recommendations, many patients suffering from progressive MS are left holding the bag, as many SPMS patients and most PPMS patients don’t exhibit signs of active inflammatory disease (enhancing lesions), and thus likely would not be considered good candidates for HSCT. Eligibility for the treatment cannot be ascertained by disability levels alone, as the majority of PPMS patients never display signs of active inflammation (enhancing lesions) even when first presenting with the disease, when disability levels are in many cases barely detectable.
Throughout the world of MS research there is a growing recognition that early treatment is the key to reducing the impact of multiple sclerosis. In fact, many researchers and clinicians now talk of a window of opportunity before the disease becomes entrenched, when all treatment options have their best chance of success. This is the time when the immune system plays its most active role in the disease, and when enhancing lesions are most likely to be rampant. Because HSCT is not without risk and can be a difficult process to get through, it may be challenging for doctors and patients alike to be convinced that this treatment may be their best chance at diminishing the long-term physical impact of multiple sclerosis before that window of opportunity is missed. In fact, it may be a mindset that sees HSCT as too draconian that proves to be the biggest impediment to the widespread adoption of this therapeutic approach.
Despite the immense potential of HSCT, it’s important to understand at the deepest level that this is still an experimental treatment option. Although there is serious science backing the effectiveness of HSCT, protocols have yet to be completely standardized, best practices are still being ascertained, and large-scale trials are still underway (click here). HSCT has not been approved for the treatment of MS by any national or international regulatory body. Nevertheless, as might be expected, a medical tourism industry is springing up around HSCT, and more and more patients are traveling to different locations around the world to undergo the procedure. Websites and Facebook pages about HSCT are proliferating, with some spreading what at best be termed low-grade information. There is a wealth of anecdotal evidence in the form of legitimate patient testimonials confirming the effectiveness of the treatment (click here), but anecdotal evidence for any alternative treatment is almost always skewed heavily towards the positive (folks with negative experiences with such treatments, especially expensive ones, rarely post about them on the Internet), and more than a few of the Internet “resources” I’ve come across seem to be more marketing tools than reliable sources of actionable facts.
Therefore, it is absolutely essential that any patient considering HSCT educate themselves using the most scientifically legitimate resources available, and then educate themselves some more. One should never put complete trust in any patient driven source of info (this blog included), and special diligence should be taken when reading information supplied by companies and institutions offering, for a price, the treatment itself. Some of the best research papers I’ve read on HSCT can be found at the following links (click here, here, and here).
All of these caveats aside, Hematopoietic Stem Cell Transplantation could very well represent a major shift in the way MS is treated in the not-too-distant future, at least for a substantial subset of patients afflicted with the disease. HSCT is serious business, but so too is multiple sclerosis. Even though studies suggest that HSCT does not put a permanent stop to the disease (click here), the promise of many years of life free from any symptoms is enormously tantalizing, so much so that many patients are proving to be more than willing to take the plunge. One can only hope that as researchers perfect their skills and methodologies HSCT will become ever more safe and effective. While likely not the Holy Grail of a cure, HSCT could represent a significant step forward in treating this very ugly disease, albeit one that apparently and unfortunately has little to offer many of those most disabled by the illness, those stuck in the stranglehold of progressive MS whose disease is absent active inflammation.
The below video is a segment from the Australian version of the TV news magazine 60 Minutes, which traces an Aussie MS patient’s successful HSCT journey to Russia and back. Please note that this is not in any way an endorsement of the Russian clinic offering the treatment. In fact, as I was writing this article, news landed in my email inbox of a patient who died at this Moscow clinic while undergoing HSCT for the treatment of a rare disease called Stiff Person Syndrome (click here). Though the news states that HSCT was not necessarily the direct cause of her death, this should only emphasize the serious nature of this treatment regimen. Again, patient selection is the key ingredient to the success of this treatment. Patients must be physically strong enough to endure the taxing effects of the chemotherapy agents used during HSCT, the toxicity of which varies from treatment center to treatment center. One must guard against letting hope eclipse reason, but I know all too well that desperate times can call for desperate measures…
Once the immune system has been eradicated, the previously harvested stem cells are intravenously infused back into the patient’s body, where over the course of several weeks they rebuild the immune system, effectively giving the patient an entirely new array of immune cells. In theory, this brand-new immune system shouldn’t have the destructive tendencies that led their old immune cells to attack the patient’s own central nervous system tissues, the mechanism that is believed to cause the damage and lesions that give multiple sclerosis its name.
Though this may seem like a sledgehammer approach to treating MS, recent studies have shown HSCT to be astoundingly effective when used on properly selected patients. One recently published study followed 52 Swedish MS patients that were treated with HSCT (click here). At five years, relapse free survival was 87%, MRI event free survival 85%, EDSS score progression free survival 77%, and disease-free survival (no relapses, no new MRI lesions, and no EDSS progression) was 68%. The presence of Gadolinium enhancing lesions prior to HSCT was associated with a significantly higher degree of favorable outcome (79% exhibiting disease-free survival at five years). The study’s authors conclude that that “HSCT is a very effective treatment of inflammatory active MS and can be performed with a high degree of safety at experienced centers.” Other recent studies looking at the efficacy of HSCT have found similar results (click here and here). Outcomes such as these give ample reason to sit up and take notice, despite the admittedly frightening prospect of the use of intense chemotherapy conditioning regimens.
One must keep in mind that despite recent advances in treating MS with a new generation of immunosuppressant drugs, the disease in its most serious forms remains a brutal beast intent on laying waste to many of those it afflicts. Next generation MS drugs such as Tysabri, Gilenya, and Tecfidera are proving to be potent in managing the disease in those patients on whom they are effective, but these drugs must be taken indefinitely and each has their own set of possible serious side effects that give many patients pause. HSCT, on the other hand, is meant to be a one-time treatment, after which properly selected and treated patients are proving to show a remarkable degree of sustained disease-free existence, with some even experiencing a reversal in disability status (click here). While no responsible person is calling HSCT a cure for MS, and undergoing the treatment is no walk in the park (intense chemotherapy is serious business), years of disease-free life without the indefinite use of drugs is the stuff of most MSer’s dreams.
HSCT has been proven to work best on patients with very active inflammatory disease (those “properly selected patients” I keep talking about), meaning patients whose disease is marked by frequent relapses and enhancing lesions on their MRI images. Many if not most RRMS patients fall into this category, but unfortunately far fewer SPMS and PPMS patients fit the bill. Study after study (click here and here) has shown that the most important criteria for identifying patients on whom HSCT has the best chance of success is the presence of enhancing lesions as detected by MRI.
Enhancing lesions are a sign that the immune system is actively causing inflammation within a patient’s central nervous system, while the lack of enhancing lesions signals that the disease is being driven by some other, as yet undiscovered, mechanism. A comprehensive overview of HSCT results on MS patients worldwide conducted by Brazilian researchers came up with the following recommendations for selecting patients who might benefit from HSCT: “… the forms of the disease that might benefit from transplantation are: relapsing remitting, primary or secondary progressive, and the “malignant” form, provided there is evidence of inflammatory activity at the time of transplant indication.” These researchers further suggest that the treatment not be given to patients who have lost the ability to walk, with the exception of those suffering from extremely aggressive MS who have accumulated disability quickly. It’s also very important that patients be relatively healthy aside from their MS, hearty enough to withstand the rigors of a short burst of intense chemotherapy.
Unfortunately, as is illustrated by the above recommendations, many patients suffering from progressive MS are left holding the bag, as many SPMS patients and most PPMS patients don’t exhibit signs of active inflammatory disease (enhancing lesions), and thus likely would not be considered good candidates for HSCT. Eligibility for the treatment cannot be ascertained by disability levels alone, as the majority of PPMS patients never display signs of active inflammation (enhancing lesions) even when first presenting with the disease, when disability levels are in many cases barely detectable.
Throughout the world of MS research there is a growing recognition that early treatment is the key to reducing the impact of multiple sclerosis. In fact, many researchers and clinicians now talk of a window of opportunity before the disease becomes entrenched, when all treatment options have their best chance of success. This is the time when the immune system plays its most active role in the disease, and when enhancing lesions are most likely to be rampant. Because HSCT is not without risk and can be a difficult process to get through, it may be challenging for doctors and patients alike to be convinced that this treatment may be their best chance at diminishing the long-term physical impact of multiple sclerosis before that window of opportunity is missed. In fact, it may be a mindset that sees HSCT as too draconian that proves to be the biggest impediment to the widespread adoption of this therapeutic approach.
Despite the immense potential of HSCT, it’s important to understand at the deepest level that this is still an experimental treatment option. Although there is serious science backing the effectiveness of HSCT, protocols have yet to be completely standardized, best practices are still being ascertained, and large-scale trials are still underway (click here). HSCT has not been approved for the treatment of MS by any national or international regulatory body. Nevertheless, as might be expected, a medical tourism industry is springing up around HSCT, and more and more patients are traveling to different locations around the world to undergo the procedure. Websites and Facebook pages about HSCT are proliferating, with some spreading what at best be termed low-grade information. There is a wealth of anecdotal evidence in the form of legitimate patient testimonials confirming the effectiveness of the treatment (click here), but anecdotal evidence for any alternative treatment is almost always skewed heavily towards the positive (folks with negative experiences with such treatments, especially expensive ones, rarely post about them on the Internet), and more than a few of the Internet “resources” I’ve come across seem to be more marketing tools than reliable sources of actionable facts.
Therefore, it is absolutely essential that any patient considering HSCT educate themselves using the most scientifically legitimate resources available, and then educate themselves some more. One should never put complete trust in any patient driven source of info (this blog included), and special diligence should be taken when reading information supplied by companies and institutions offering, for a price, the treatment itself. Some of the best research papers I’ve read on HSCT can be found at the following links (click here, here, and here).
All of these caveats aside, Hematopoietic Stem Cell Transplantation could very well represent a major shift in the way MS is treated in the not-too-distant future, at least for a substantial subset of patients afflicted with the disease. HSCT is serious business, but so too is multiple sclerosis. Even though studies suggest that HSCT does not put a permanent stop to the disease (click here), the promise of many years of life free from any symptoms is enormously tantalizing, so much so that many patients are proving to be more than willing to take the plunge. One can only hope that as researchers perfect their skills and methodologies HSCT will become ever more safe and effective. While likely not the Holy Grail of a cure, HSCT could represent a significant step forward in treating this very ugly disease, albeit one that apparently and unfortunately has little to offer many of those most disabled by the illness, those stuck in the stranglehold of progressive MS whose disease is absent active inflammation.
The below video is a segment from the Australian version of the TV news magazine 60 Minutes, which traces an Aussie MS patient’s successful HSCT journey to Russia and back. Please note that this is not in any way an endorsement of the Russian clinic offering the treatment. In fact, as I was writing this article, news landed in my email inbox of a patient who died at this Moscow clinic while undergoing HSCT for the treatment of a rare disease called Stiff Person Syndrome (click here). Though the news states that HSCT was not necessarily the direct cause of her death, this should only emphasize the serious nature of this treatment regimen. Again, patient selection is the key ingredient to the success of this treatment. Patients must be physically strong enough to endure the taxing effects of the chemotherapy agents used during HSCT, the toxicity of which varies from treatment center to treatment center. One must guard against letting hope eclipse reason, but I know all too well that desperate times can call for desperate measures…
![Cover of "Gojira [Blu-ray]"](http://ecx.images-amazon.com/images/I/51luMbSrBWL._SL350_.jpg)
