With growing momentum, stem cell therapies of all types are creating a huge buzz in the MS community. While one form of stem cell therapy – that intended to regenerate damaged nervous system tissue – is clearly still in its experimental infancy, another, hematopoietic stem cell therapy, which seeks to reboot the immune system, has been used on MS patients for almost 20 years. Early attempts at using HSCT to treat MS, though at times producing encouraging results, were fraught with danger, with as many as 10% of test subjects dying as a direct result of the procedure. Recent refinements in technique, better patient selection, and a growing knowledge base are now bringing HSCT closer to mainstream use as efficacy rates have soared and mortality rates have dropped dramatically. In the case of the best treatment centers, mortality rates have dropped below 1%, with no deaths reported over the last five or so years.
It’s very important to understand that there are currently two wholly separate and completely different approaches being explored for using stem cells to treat multiple sclerosis. Both hold tremendous promise but go about their business in entirely different ways, and care must be taken to never confuse the two. Regenerative stem cell therapies, almost all using some form of mesenchymal stem cells, seek to repair brain and spine tissues damaged by MS, while HSCT is focused entirely on the immune system and does not directly address damaged nervous system tissues at all. This post will deal exclusively with HSCT. For more info on the differences between these two stem cell methodologies, and more details on the experimental regenerative therapies, please refer to a previous overview of stem cell therapies for MS that appeared on Wheelchair Kamikaze last fall (click here).
So, first things first – what exactly is HSCT? In practice, HSCT is very similar to the bone marrow transplants that have been used to treat patients with leukemia and other cancers of the blood for decades. As a therapy for multiple sclerosis, the process begins by collecting a patient’s own stem cells, either through bone marrow harvesting or blood draws. Once collected, these stem cells are stored, and sometimes multiplied, in sterile laboratory conditions.
Then comes the dramatic part – the patient’s immune system is ablated (a polite way of saying destroyed) over the course of several days using powerful chemotherapy agents. Depending on the clinic treating the patient, a variety of drugs or combination of drugs is used, some more intense than others. The goal of this “conditioning regimen” is to leave the subject with no functioning immune system, obviously a very vulnerable state during which the patient must be kept in isolation to prevent exposure to any possible contaminants or infectious agents. While undergoing this conditioning regimen, patients typically suffer many of the common side effects of chemotherapy, including hair loss and nausea.
Once the immune system has been eradicated, the previously harvested stem cells are intravenously infused back into the patient’s body, where over the course of several weeks they rebuild the immune system, effectively giving the patient an entirely new array of immune cells. In theory, this brand-new immune system shouldn’t have the destructive tendencies that led their old immune cells to attack the patient’s own central nervous system tissues, the mechanism that is believed to cause the damage and lesions that give multiple sclerosis its name.
Though this may seem like a sledgehammer approach to treating MS, recent studies have shown HSCT to be astoundingly effective when used on properly selected patients. One recently published study followed 52 Swedish MS patients that were treated with HSCT (click here). At five years, relapse free survival was 87%, MRI event free survival 85%, EDSS score progression free survival 77%, and disease-free survival (no relapses, no new MRI lesions, and no EDSS progression) was 68%. The presence of Gadolinium enhancing lesions prior to HSCT was associated with a significantly higher degree of favorable outcome (79% exhibiting disease-free survival at five years). The study’s authors conclude that that “HSCT is a very effective treatment of inflammatory active MS and can be performed with a high degree of safety at experienced centers.” Other recent studies looking at the efficacy of HSCT have found similar results (click here and here). Outcomes such as these give ample reason to sit up and take notice, despite the admittedly frightening prospect of the use of intense chemotherapy conditioning regimens.
One must keep in mind that despite recent advances in treating MS with a new generation of immunosuppressant drugs, the disease in its most serious forms remains a brutal beast intent on laying waste to many of those it afflicts. Next generation MS drugs such as Tysabri, Gilenya, and Tecfidera are proving to be potent in managing the disease in those patients on whom they are effective, but these drugs must be taken indefinitely and each has their own set of possible serious side effects that give many patients pause. HSCT, on the other hand, is meant to be a one-time treatment, after which properly selected and treated patients are proving to show a remarkable degree of sustained disease-free existence, with some even experiencing a reversal in disability status (click here). While no responsible person is calling HSCT a cure for MS, and undergoing the treatment is no walk in the park (intense chemotherapy is serious business), years of disease-free life without the indefinite use of drugs is the stuff of most MSer’s dreams.
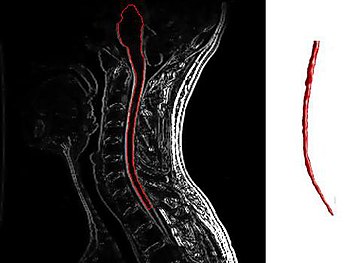
HSCT has been proven to work best on patients with very active inflammatory disease (those “properly selected patients” I keep talking about), meaning patients whose disease is marked by frequent relapses and enhancing lesions on their MRI images. Many if not most RRMS patients fall into this category, but unfortunately far fewer SPMS and PPMS patients fit the bill. Study after study (click here and here) has shown that the most important criteria for identifying patients on whom HSCT has the best chance of success is the presence of enhancing lesions as detected by MRI.
Enhancing lesions are a sign that the immune system is actively causing inflammation within a patient’s central nervous system, while the lack of enhancing lesions signals that the disease is being driven by some other, as yet undiscovered, mechanism. A comprehensive overview of HSCT results on MS patients worldwide conducted by Brazilian researchers came up with the following recommendations for selecting patients who might benefit from HSCT: “… the forms of the disease that might benefit from transplantation are: relapsing remitting, primary or secondary progressive, and the “malignant” form, provided there is evidence of inflammatory activity at the time of transplant indication.” These researchers further suggest that the treatment not be given to patients who have lost the ability to walk, with the exception of those suffering from extremely aggressive MS who have accumulated disability quickly. It’s also very important that patients be relatively healthy aside from their MS, hearty enough to withstand the rigors of a short burst of intense chemotherapy.
Unfortunately, as is illustrated by the above recommendations, many patients suffering from progressive MS are left holding the bag, as many SPMS patients and most PPMS patients don’t exhibit signs of active inflammatory disease (enhancing lesions), and thus likely would not be considered good candidates for HSCT. Eligibility for the treatment cannot be ascertained by disability levels alone, as the majority of PPMS patients never display signs of active inflammation (enhancing lesions) even when first presenting with the disease, when disability levels are in many cases barely detectable.
Throughout the world of MS research there is a growing recognition that early treatment is the key to reducing the impact of multiple sclerosis. In fact, many researchers and clinicians now talk of a window of opportunity before the disease becomes entrenched, when all treatment options have their best chance of success. This is the time when the immune system plays its most active role in the disease, and when enhancing lesions are most likely to be rampant. Because HSCT is not without risk and can be a difficult process to get through, it may be challenging for doctors and patients alike to be convinced that this treatment may be their best chance at diminishing the long-term physical impact of multiple sclerosis before that window of opportunity is missed. In fact, it may be a mindset that sees HSCT as too draconian that proves to be the biggest impediment to the widespread adoption of this therapeutic approach.
Despite the immense potential of HSCT, it’s important to understand at the deepest level that this is still an experimental treatment option. Although there is serious science backing the effectiveness of HSCT, protocols have yet to be completely standardized, best practices are still being ascertained, and large-scale trials are still underway (click here). HSCT has not been approved for the treatment of MS by any national or international regulatory body. Nevertheless, as might be expected, a medical tourism industry is springing up around HSCT, and more and more patients are traveling to different locations around the world to undergo the procedure. Websites and Facebook pages about HSCT are proliferating, with some spreading what at best be termed low-grade information. There is a wealth of anecdotal evidence in the form of legitimate patient testimonials confirming the effectiveness of the treatment (click here), but anecdotal evidence for any alternative treatment is almost always skewed heavily towards the positive (folks with negative experiences with such treatments, especially expensive ones, rarely post about them on the Internet), and more than a few of the Internet “resources” I’ve come across seem to be more marketing tools than reliable sources of actionable facts.
Therefore, it is absolutely essential that any patient considering HSCT educate themselves using the most scientifically legitimate resources available, and then educate themselves some more. One should never put complete trust in any patient driven source of info (this blog included), and special diligence should be taken when reading information supplied by companies and institutions offering, for a price, the treatment itself. Some of the best research papers I’ve read on HSCT can be found at the following links (click here, here, and here).
All of these caveats aside, Hematopoietic Stem Cell Transplantation could very well represent a major shift in the way MS is treated in the not-too-distant future, at least for a substantial subset of patients afflicted with the disease. HSCT is serious business, but so too is multiple sclerosis. Even though studies suggest that HSCT does not put a permanent stop to the disease (click here), the promise of many years of life free from any symptoms is enormously tantalizing, so much so that many patients are proving to be more than willing to take the plunge. One can only hope that as researchers perfect their skills and methodologies HSCT will become ever more safe and effective. While likely not the Holy Grail of a cure, HSCT could represent a significant step forward in treating this very ugly disease, albeit one that apparently and unfortunately has little to offer many of those most disabled by the illness, those stuck in the stranglehold of progressive MS whose disease is absent active inflammation.
The below video is a segment from the Australian version of the TV news magazine 60 Minutes, which traces an Aussie MS patient’s successful HSCT journey to Russia and back. Please note that this is not in any way an endorsement of the Russian clinic offering the treatment. In fact, as I was writing this article, news landed in my email inbox of a patient who died at this Moscow clinic while undergoing HSCT for the treatment of a rare disease called Stiff Person Syndrome (click here). Though the news states that HSCT was not necessarily the direct cause of her death, this should only emphasize the serious nature of this treatment regimen. Again, patient selection is the key ingredient to the success of this treatment. Patients must be physically strong enough to endure the taxing effects of the chemotherapy agents used during HSCT, the toxicity of which varies from treatment center to treatment center. One must guard against letting hope eclipse reason, but I know all too well that desperate times can call for desperate measures…
Once the immune system has been eradicated, the previously harvested stem cells are intravenously infused back into the patient’s body, where over the course of several weeks they rebuild the immune system, effectively giving the patient an entirely new array of immune cells. In theory, this brand-new immune system shouldn’t have the destructive tendencies that led their old immune cells to attack the patient’s own central nervous system tissues, the mechanism that is believed to cause the damage and lesions that give multiple sclerosis its name.
Though this may seem like a sledgehammer approach to treating MS, recent studies have shown HSCT to be astoundingly effective when used on properly selected patients. One recently published study followed 52 Swedish MS patients that were treated with HSCT (click here). At five years, relapse free survival was 87%, MRI event free survival 85%, EDSS score progression free survival 77%, and disease-free survival (no relapses, no new MRI lesions, and no EDSS progression) was 68%. The presence of Gadolinium enhancing lesions prior to HSCT was associated with a significantly higher degree of favorable outcome (79% exhibiting disease-free survival at five years). The study’s authors conclude that that “HSCT is a very effective treatment of inflammatory active MS and can be performed with a high degree of safety at experienced centers.” Other recent studies looking at the efficacy of HSCT have found similar results (click here and here). Outcomes such as these give ample reason to sit up and take notice, despite the admittedly frightening prospect of the use of intense chemotherapy conditioning regimens.
One must keep in mind that despite recent advances in treating MS with a new generation of immunosuppressant drugs, the disease in its most serious forms remains a brutal beast intent on laying waste to many of those it afflicts. Next generation MS drugs such as Tysabri, Gilenya, and Tecfidera are proving to be potent in managing the disease in those patients on whom they are effective, but these drugs must be taken indefinitely and each has their own set of possible serious side effects that give many patients pause. HSCT, on the other hand, is meant to be a one-time treatment, after which properly selected and treated patients are proving to show a remarkable degree of sustained disease-free existence, with some even experiencing a reversal in disability status (click here). While no responsible person is calling HSCT a cure for MS, and undergoing the treatment is no walk in the park (intense chemotherapy is serious business), years of disease-free life without the indefinite use of drugs is the stuff of most MSer’s dreams.
HSCT has been proven to work best on patients with very active inflammatory disease (those “properly selected patients” I keep talking about), meaning patients whose disease is marked by frequent relapses and enhancing lesions on their MRI images. Many if not most RRMS patients fall into this category, but unfortunately far fewer SPMS and PPMS patients fit the bill. Study after study (click here and here) has shown that the most important criteria for identifying patients on whom HSCT has the best chance of success is the presence of enhancing lesions as detected by MRI.
Enhancing lesions are a sign that the immune system is actively causing inflammation within a patient’s central nervous system, while the lack of enhancing lesions signals that the disease is being driven by some other, as yet undiscovered, mechanism. A comprehensive overview of HSCT results on MS patients worldwide conducted by Brazilian researchers came up with the following recommendations for selecting patients who might benefit from HSCT: “… the forms of the disease that might benefit from transplantation are: relapsing remitting, primary or secondary progressive, and the “malignant” form, provided there is evidence of inflammatory activity at the time of transplant indication.” These researchers further suggest that the treatment not be given to patients who have lost the ability to walk, with the exception of those suffering from extremely aggressive MS who have accumulated disability quickly. It’s also very important that patients be relatively healthy aside from their MS, hearty enough to withstand the rigors of a short burst of intense chemotherapy.
Unfortunately, as is illustrated by the above recommendations, many patients suffering from progressive MS are left holding the bag, as many SPMS patients and most PPMS patients don’t exhibit signs of active inflammatory disease (enhancing lesions), and thus likely would not be considered good candidates for HSCT. Eligibility for the treatment cannot be ascertained by disability levels alone, as the majority of PPMS patients never display signs of active inflammation (enhancing lesions) even when first presenting with the disease, when disability levels are in many cases barely detectable.
Throughout the world of MS research there is a growing recognition that early treatment is the key to reducing the impact of multiple sclerosis. In fact, many researchers and clinicians now talk of a window of opportunity before the disease becomes entrenched, when all treatment options have their best chance of success. This is the time when the immune system plays its most active role in the disease, and when enhancing lesions are most likely to be rampant. Because HSCT is not without risk and can be a difficult process to get through, it may be challenging for doctors and patients alike to be convinced that this treatment may be their best chance at diminishing the long-term physical impact of multiple sclerosis before that window of opportunity is missed. In fact, it may be a mindset that sees HSCT as too draconian that proves to be the biggest impediment to the widespread adoption of this therapeutic approach.
Despite the immense potential of HSCT, it’s important to understand at the deepest level that this is still an experimental treatment option. Although there is serious science backing the effectiveness of HSCT, protocols have yet to be completely standardized, best practices are still being ascertained, and large-scale trials are still underway (click here). HSCT has not been approved for the treatment of MS by any national or international regulatory body. Nevertheless, as might be expected, a medical tourism industry is springing up around HSCT, and more and more patients are traveling to different locations around the world to undergo the procedure. Websites and Facebook pages about HSCT are proliferating, with some spreading what at best be termed low-grade information. There is a wealth of anecdotal evidence in the form of legitimate patient testimonials confirming the effectiveness of the treatment (click here), but anecdotal evidence for any alternative treatment is almost always skewed heavily towards the positive (folks with negative experiences with such treatments, especially expensive ones, rarely post about them on the Internet), and more than a few of the Internet “resources” I’ve come across seem to be more marketing tools than reliable sources of actionable facts.
Therefore, it is absolutely essential that any patient considering HSCT educate themselves using the most scientifically legitimate resources available, and then educate themselves some more. One should never put complete trust in any patient driven source of info (this blog included), and special diligence should be taken when reading information supplied by companies and institutions offering, for a price, the treatment itself. Some of the best research papers I’ve read on HSCT can be found at the following links (click here, here, and here).
All of these caveats aside, Hematopoietic Stem Cell Transplantation could very well represent a major shift in the way MS is treated in the not-too-distant future, at least for a substantial subset of patients afflicted with the disease. HSCT is serious business, but so too is multiple sclerosis. Even though studies suggest that HSCT does not put a permanent stop to the disease (click here), the promise of many years of life free from any symptoms is enormously tantalizing, so much so that many patients are proving to be more than willing to take the plunge. One can only hope that as researchers perfect their skills and methodologies HSCT will become ever more safe and effective. While likely not the Holy Grail of a cure, HSCT could represent a significant step forward in treating this very ugly disease, albeit one that apparently and unfortunately has little to offer many of those most disabled by the illness, those stuck in the stranglehold of progressive MS whose disease is absent active inflammation.
The below video is a segment from the Australian version of the TV news magazine 60 Minutes, which traces an Aussie MS patient’s successful HSCT journey to Russia and back. Please note that this is not in any way an endorsement of the Russian clinic offering the treatment. In fact, as I was writing this article, news landed in my email inbox of a patient who died at this Moscow clinic while undergoing HSCT for the treatment of a rare disease called Stiff Person Syndrome (click here). Though the news states that HSCT was not necessarily the direct cause of her death, this should only emphasize the serious nature of this treatment regimen. Again, patient selection is the key ingredient to the success of this treatment. Patients must be physically strong enough to endure the taxing effects of the chemotherapy agents used during HSCT, the toxicity of which varies from treatment center to treatment center. One must guard against letting hope eclipse reason, but I know all too well that desperate times can call for desperate measures…
Hi Marc, Thank you once again for a very informative piece! Though I am sure that I would not fit the criteria myself, I applaud those pioneers who've had the courage (desperation?) to go for it and in doing so providing more hope for the future. I so enjoy your blog!
ReplyDeleteHope that you & Karen are well!
Karen
Hi Karen, thanks for commenting. Quite honestly, knowing what I know about the procedure and the beast, I think HSCT might be my first treatment choice if I was diagnosed with highly active MS. Might as well go for the gusto, and give yourself the best chance of not having to deal with MS symptoms for years at a time. That said, everybody's tolerance for risk is different, and this is a rather draconian treatment. I suppose a deciding factor would be how hard the disease hits early on. As HSCT techniques become more refined, I think the choice will become even easier still…
DeleteMarc,
ReplyDeleteA very well written piece on HSCT. Fair and balanced. I happen to know a bit more than most about this treatment. In December of last year, I was treated by Dr. Burt and his amazing team up at Northwestern in Chicago. I would like to thank them for giving me my future back. Thank you for helping to spread the word about this amazing new tool in fighting the monster that is MS.
Hello Marc, my name is CoCo. are you saying you were treated with HSCT in Chicago? if so could you please email me your doctor's phone number? I would love to have a chance for a future because I live in a state of depression. My whole quality of life has changed. Although I have RR MS, and get through life on a crutch I truly rather be dead. Like you, I would love to have a chance for a future. My email is Relapprais@aol.com. Thank you.
DeleteI'm sorry, this is "Start Glaze". I meant to pose my questions to Keith Ludwig.
DeleteKeith, very happy that the procedure worked so well for you. HSCT really could be a game changer for many patients, and word about it should spread. Folks have to be careful about using which treatment center to go to, though, as not all are equally proficient at this transplant therapy, so doing your due diligence is a must. Thanks for your comments, and best of luck going forward.
DeleteStarr, the name of the Dr. doing HSCT in Chicago is Dr. Burt, at Northwestern University. A quick Google should get you the required info.
Perhaps something in the horizon for PPMS too?
ReplyDeletehttp://www.sciencedaily.com/releases/2014/07/140724182929.htm
Yes, this is promising news. Keep in mind this is probably most useful for the regenerative therapies, though. Completely different than HSCT.
DeleteMarc, we are in your debt for this thorough and balanced portrayal of this treatment option. Thank you. The MS community is so lucky to have you.
ReplyDeleteThanks so much, Judy. It really helps to know that I'm doing my part to help other people with this freaking disease. I just wish some of my research would finally turn up a treatment that unequivocally is effective for us progressive types…
DeleteYour timing is prescient, Marc, as it often is. As a clinical trial HSCT participant in 2010 (it was a success—no drugs or progression to date), I have to say your information is spot-on.
ReplyDeleteHello Dave,
DeleteWhere did you do your clinical trial?
Dave, it's terrific that HSCT was a triumph for you. At the risk of sounding like a complete nerd, may the force be with you. Honestly, I've only ever seen the first Star Wars movie. I know that kind of makes me a freak these days. What can I say?
DeleteStarr: Houston's MD Anderson Cancer Center. Part of HALT-MS.
DeleteMarc, may the force be with you as well.
DeleteExcellent piece!
ReplyDeleteThanks, Daphne…
Delete"While no responsible person is calling HSCT a cure for MS"? If HSCT halts MS, would that not be considered a cure to you?
ReplyDeleteNo, a cure would mean complete eradication of disease. HSCT halts the disease for some patients, but according to most studies, not indefinitely. Most studies have only looked at patients up to seven years after treatment. The few that have looked further have found disease progression resumes in many patients. Additionally, even after HSCT patients display O-bands in their cerebrospinal fluid, indicating some kind of disease process is likely still going on.
DeleteThat said, perhaps as the technique becomes more refined and evolved, results will start to resemble a cure. MS is such a weird beast, and presents itself in so many different ways, that it would actually be difficult to nail down the definition of a cure. That said, I'm sure almost any MS patient would happily (maybe even greedily) take five, six, or seven years plus of disease-free living. The fact that HSCT reverses disability in some patients is absolutely incredible, which is why the treatment should gain widespread attention from patients and physicians alike.
Thank you. George Goss in the HSCT forums paints a much different picture. He claims this is a cure and very defensive about this in the past. He also never really gives us the numbers of people where the MS has returned but instead posts pictures of charts. I am a member of all 3 HSCT forums on FB. It seems like so many are running off to have this treatment.
DeleteI agree that all patients should do their due diligence and read as much research on this topic as possible. The paper you cite and give a link to provides conflicting information. While in some parts it suggests that HSCT is effective in treating PPMS, the authors conclude, in part, that "such resetting of the immune system is only effective in the early stages of MS, particularly in relapsing remitting MS."
ReplyDeleteHow/is this any different from the work/treatments done at John Hopkins some years back ? I think the treatment was with hiCy and Peter Caslabresi was one of the investigators. It was not touted as a 'cure all' and I think bladder cancer was a long term risk but it sounded good to me back then. Are they just doing the same process but with a more selective patient group? I think I remember something about people going to Cost Rica for treatment, but maybe that was just the stem cell part, without the chemo? I'm not sure.
ReplyDeleteThanks for keeping us all in the MS loop.
HSCT is similar to HyCy, which was known as Revimmune back when it was being trialed at Johns Hopkins. Revimmune knocked out the immune system using high doses of the chemotherapy drug cyclophosphamide, and it didn't damage the bone marrow so replacement stem cells weren't necessary. Patients would simply go through intensive cyclophosphamide treatments, which were then followed by drugs that stimulated the bone marrow to produce new immune system cells. It seems the Hopkins trials were successful for many patients, although I know a few that went through the treatment only to see their disease bounce back. These were fairly advanced SPMS patients who still slowed signs of active inflammatory disease. Hopkins ran out of money to fund the trials, and another company bought the rights to the treatment protocol. I don't think any more research has been done, however, because of further lack of funding. Cyclophosphamide is a cheap drug, so the pharmaceutical companies aren't interested in funding trials. An unfortunate reality given our current medical research model.
DeleteI tried to get in on the Johns Hopkins trials when they were first announced, but was rejected because I didn't have any enhancing lesions. Was quite bummed about that at the time.
The treatment being offered in Costa Rica is regenerative stem cell therapy using mesenchymal stem cells derived from a patient's own fat tissues. I've known a few people who have gone down to Costa Rica (the clinic has now moved to Panama), but didn't see very good results.
Marc,
DeleteThanks. It is encouraging to see the improvements of the treatment being refined. And hopefully they'll get it perfected soon. Time is everything with MS, it seems.
I've had MS for 22 years. Neurologist says I I'm not progressive yet but I skipped the gadolinium last MRI so as not to disrupt breastfeeding. But I had a relapse while pregnant so I'm hoping that says I'm still RR.
I've been looking to find the earliest patients and the long term benefits/side effects they have experienced. Like did they hit progressive anyway but at least with less disability than they would have? think I read leukemia was a possibility down the road, not sure the odds or timing. Is there such a collection of data?
its so maddening to feel there is a cliff you might not recover from somewhere in you future and helpless to do anything about it.
though not perfect condition now, if I could have a shot at maintaining the same for the next 20 years I would be thrilled.
As others have said, thanks again for such a unbiased and thorough post!
ReplyDeleteAs someone who has a somewhat less severe case (still able to walk a fair distance without assistance after 10 years), I struggle with what to do. The disease is slowly taking from me (I can no longer run, play sports, go to a theme park, etc.), but not so much that I feel the need to seek out "drastic" measures. Yet I fear for what the future may bring and, of course, wish I could do the things I used to. If there is a reasonable therapy that could stop it in it's tracks, or even better, reverse some of the damage, I am all ears.
Meanwhile, I feel stuck in the middle - not bad enough to take drastic measures, but still feeling the effects of this disease on a daily basis and fearful of what may come next. I keep reading about trials and people who have experienced dramatic improvements with HSCT, Stem Cell therapy (no chemo) and HSCT - and I am here on the sidelines, confused and doing nothing.
Maybe I am just impatient???
This comment has been removed by the author.
DeleteOops - I meant HSCT, Stem Cell therapy, and CCSVI...
DeleteHi Jeff, not knowing the specific of your case, it's very difficult to make any tangible suggestions. However, it does sound like the disease has stolen quite a bit from you, even though your progression has been relatively slow (from some patients perspective, your case may not seem very slow at all). Your treatment options are by necessity dictated by your clinical and diagnostic picture. In other words, whether your disease is relapsing or progressing, whether your MRIs show enhancing lesions, etc. If you do have "active" disease (meaning enhancing lesions and/or relapses) then a wide range of treatment options are open to you, including the newer generation disease modifying drugs, as well as more experimental treatments such as HSCT. If you are suffering from strictly progressive disease then these options offer much less promise.
DeleteEverybody has a different tolerance for risk, and a different level of aggressiveness in combating their disease. I've always been of the mindset that doing nothing is simply not an option. I pretty much know where doing nothing is going to lead, and it's not any place I care to visit. Having said that, none of the myriad treatment options I have tried has done much of anything to impede the progression of my disease, but I personally feel better doing something than nothing, even if that something proves to be ineffective.
In all honesty, if I had any sign of active (enhancing) lesions, I would very, very seriously consider HSCT. The other therapies you mention, while holding varying degrees of promise, have nowhere near the documented efficacy of HSCT, although they are considerably less "draconian".
This is a discussion you must have with your neurologist. If for some reason you don't feel comfortable discussing this with your neurologist, or he's not open to discussing treatment options with you for whatever reason, my advice would be to find a new neurologist. Yes, many neurologists will pooh-pooh some of these alternative treatments, but at the very least you should get the complete clinical picture of your particular flavor of the disease, from which you can then make some educated decisions.
Wishing you the best…
Thanks for the reply Marc.
DeleteI should have mentioned that I am now on Tecfidera (3 months in), so I guess the statement that I am doing nothing was not quite correct. I just had a brain MRI and when compared to the previous one (3 years ago) it showed no new lesions. Good news, I guess, but the problem is that I have experienced increased "disability" in the last 3 years, resulting in increased fatigue, walking/balance issues, and incontinence. Like all of us, I want to stop this monster and I'll do whatever it takes. My neuro is great, speaks at local MS Society events and keeps up with things. Although, he definitely seems to think I should wait until trials are complete and there is a proven treatment. I don't know if I want to wait another three to five (???) years, while I continue to suffer losses.
BTW, just wanted to say thank you for your dedication to this blog. I continually rely on your posts as a source of inspiration and great information. I have bookmarked your post "The Tao of MS" and read it often when I find myself letting things dictate how I feel.
Jeff I considered commenting with pretty much the same thing (wife's ms is less severe). Her lesions are a-typical for MS (I guess they're long and more to the side, something along those lines) and initial symptom was massive migraine so I'm always kind of searching for an alternative diagnosis. Currently waiting on igenex lyme test. The whole lyme controversy thing is such a mess, but figured it's worth at least testing anyway.
DeleteAnyway, I it's kind of weird to be grateful to have less severe MS (still sucks!), pretty much making some treatments not worth it...
Did you ever get anywhere with Lyme? Just in case you didn't, you should know the Igenex blood test is bit reliable, even though their testing is considered one of the better ones. There a newer test called PCR which is a urine test where they use culture to find the sneaky little bugger. It's highly successful in finding chronic Lyme, which will never be found doing western blot style blood testing. There's likely millions living with Lyme who don't think they have it. From what I've read the Lyme spirochete cannot be killed with ablative chemo (yeah its that nasty) which may explain why some HSCT patients relapse. The 'experts' may like to say that it's 'genetics' but when the person has zero MS in any family members history, how can that be correct? That's why there's a growing group that believe MS and god knows how many other so called auto immune diseases are actually chronic bacterial infections. A recent story on Kris Kristofferson's miraculous recovery from Alzheimer's (it turned out to be chronic Lyme disease) threatens to blow the lid off of what may be one of the biggest medical/pharma cover ups in history.
DeleteHi Marc,
ReplyDeleteI was a HYCy patient at Hopkins in 08'. My MS had been basically stable for 15 years then, boom, aggressive relapsing progressive. The HyCy gave me a brief reprieve and an improvement in symptoms, temporarily. In reality, it just stopped my active lesions and attacks and turned me into a strictly SP patient. It was all a mirage. By the time I had a spinal tap at IMSMP, it showed my CNS inflammation to be 27 times normal- my progression is fairly rapid- very little mobility in my legs, arms aren't great and my spinal cord shows severe atrophy. I have been having intrathecal methotrexate, but do not see any benefit. The inflammatory compounds in my CSF, however are at a much improved level since starting the methotrexate. Perhaps it has slowed down my decline. Lack of active lesions means nothing to me anymore and I don't really perceive this treatment, the chemo with stem cells, as a miracle for many of us who are the most severely affected. I wish they could figure out the cause (likely infectious) instead of plying us with these toxic Band-Aids.
Sandy
Very sorry to hear about your disease progression. Clearly, HSCT is not for everybody, and it does seem to work best on those with aggressive relapsing remitting disease.
ReplyDeleteHyCy, although successful for some patients, is a less intense version of this type of therapy. I know some other people with more advanced disease on whom the treatment did not work. Others though, did have success.
Deciding to undergo a HSCT is a serious determination. Clearly, patient selection is the key, but for the right patients the treatment has been a game changer. Unfortunately, those of us with more progressed disease don't quite fit the equation… sadly, there do seem to be some medical tourism sites that appear to accept just about any applicant. This is just plain wrong.
Wishing you the best, certainly hope the methotrexate puts the brakes on your illness…
Hi, I've been following the Kamikaze blog since I was diagnosed with MS in 2007. Your articles have been a balanced source of information, hope and inspiration. This article is especially good. I know because I am one of the HSCT success stories from Northwestern University. I did the procedure 7 months ago. It saved my life from a very aggressive Tysabri rebound attack on my brainstem. I'm now out of my wheelchair, off all MS drugs and learning to walk again. Every day is a bonus day. God bless us all.
ReplyDeleteThis sounds very promising! I hope, down the road, they would find a treatment suitable for every MS patients. Very informative post.
ReplyDelete