Friday, September 20, 2013
Stem Cell Treatments for Multiple Sclerosis
For patients, physicians, and researchers alike, stem cells hold the tantalizing potential of turning back the tide of multiple sclerosis, repairing damaged brains and spinal cords, and perhaps even offering something approaching a cure. There is plenty of hype surrounding stem cells, and they provide much reason for hope, but what is the reality of the current state of stem cell research for the treatment of MS?
As all patients with MS are aware, the currently available treatments do nothing to cure the disease or repair the damage that it does. At their best, today’s crop of disease modifying drugs (DMDs) quiet the disease, thereby improving the quality of life for many of the patients taking them, especially those suffering from relapsing remitting multiple sclerosis. However, many of these drugs carry with them risky side effect profiles, and though the newest compounds represent advances over their predecessors, patients are crying out for revolution, not evolution.
Stem cells could represent the revolution patients so fervently desire. Because of their ability to transform into almost any type of cell in the human body, stem cells may hold the key to achieving one of the holy grails of modern medicine, the regeneration and repair of damaged tissues. For MS patients, this could potentially mean the reversal of disability, and with it the long dreamt of disposal of wheelchairs, walkers, and canes. We are still a long way from that lofty goal, however, but the first few steps along the path to that salvation are currently being taken.
Though stem cell research is advancing in laboratories worldwide, the science of using stem cells to treat diseases in humans is still in its infancy. Because multiple sclerosis is a neurodegenerative disease, and its most prominent feature is the damage the disease does to the central nervous system, it is hoped that stem cells may hold the key to reversing the carnage wrought by the disease by facilitating the repair of damaged nerve cells. Furthermore, research has provided hints that stem cells may modulate the abnormal immune response seen in MS patients, and some researchers are even using stem cells to completely reboot the human immune system, a process that in some cases appears to stop the disease dead in its tracks.
It’s important to understand that there are two very different approaches to using stem cells in the treatment of multiple sclerosis. One approach hopes to use the cells to repair damaged nervous systems; the other uses stem cells to provide the patient with a brand-new immune system, one that theoretically will not turn against a patient’s own body. The latter approach is known as hematopoietic stem cell transplant, or HSCT, and has been used on patients in trial settings for almost two decades.
HSCT involves ablating (destroying) a patient’s existing immune system through the use of powerful chemotherapy drugs, and then intravenously infusing a patient’s own stem cells back into their body, a process depicted in the below diagram:
Once infused back into a patient’s body, the stem cells go about reconstituting their immune cells, effectively providing them with a brand-new immune system that in theory shouldn’t go to war against the patient’s own brain and spinal cord. In practice, this type of therapy has proven to be quite effective, particularly among patients with aggressive relapsing remitting disease who display a high amount of inflammation in their central nervous systems, as are evidenced by enhancing lesions seen on MRI imaging.
As you might imagine, using powerful chemotherapy drugs to destroy a patient’s immune system is not without its dangers, and early attempts at this therapy had mortality rates as high as 10%. As researchers perfected their methodology and began using less dangerous chemotherapy agents, though, the risks associated with HSCT dropped dramatically. Today, most patients undergoing HSCT are subjected to chemotherapy and immunosuppressive agents that do not completely destroy their bone marrow, and the safety profile of the procedure has improved impressively. The results achieved by this HSCT can be dramatic. In one study (click here) that looked at the long-term outcomes of HSCT, after 11 years 44% of patients who had started out with aggressive relapsing remitting disease were free from disability progression. By comparison, only 10% of those who did not display signs of active inflammation before HSCT remained stable.
One of the primary proponents of HSCT therapy for MS patients, Dr. Richard Burt of Northwestern University, stresses that the proper selection of patients is the key to the success of the treatment. In fact, the title of the paper he recently published (click here) includes the phrase “if no inflammation, no response”. “It’s the only therapy to date that has been shown to reverse neurologic deficits,” said Dr. Burt, “But you have to get the right group of patients.” In a study published by Dr. Burt in 2009, 17 out of 21 relapsing remitting patients improved after HSCT, and after three years all patients were free from progression (click here). Dr. Burt is currently heading up the HALT-MS trial for HSCT (click here). There are several centers around the world offering HSCT therapy, and there is a Worldwide HSCT Facebook group (click here) that contains information on all of the legitimate HSCT facilities worldwide. The group is populated by many folks who have undergone HSCT therapy. Be aware that it’s a private group, and you must request membership before being given access to all of the available information.
While HSCT holds much promise for putting the brakes on very aggressive relapsing remitting multiple sclerosis, it unfortunately has little to offer those with progressive disease, and does nothing to directly repair the damage done to the central nervous system by MS. Fortunately, another form of stem cell therapy proposes to do just that. Researchers in two centers in the US have received FDA approval to use bone marrow derived mesenchymal stem cells (MSCs) to repair nervous system damage, thereby possibly reversing the effects of the disease. There are additional trials using MSCs to treat MS underway internationally. Mesenchymal stem cells have the ability to transform (differentiate) into many different cell types, and could prove to be the building blocks necessary for repairing damage to the central nervous system as well as other organs and tissues. Experiments using MSCs to treat animal models of MS have been very encouraging (click here), demonstrating the cells’ abilities to modulate the immune system and spur the repair of damaged nervous system tissues. It remains to be seen whether the same effects can be achieved when using the cells to treat human beings.
The two FDA approved studies both use MSCs harvested from a patient’s own bone marrow, but employ them in very different ways. One study, currently underway at the Cleveland Clinic (click here), infuses mesenchymal stem cells intravenously into the patient, in the expectation that the cells will modulate the immune system and also initiate the regeneration of damaged tissues in the central nervous system. This study, which will eventually use MSCs to treat 24 patients, is proceeding slowly, but as the above linked to article details, one of the first patients treated is already reporting encouraging results.
The second FDA approved trial, to be conducted by the Tisch MS Research Center of New York (which just so happens to be my MS clinic), will use mesenchymal stem cells that have been transformed through a proprietary laboratory process into neural progenitor (NP) cells, injected directly into the spinal fluid (intrathecally)) of the patient (click here). Neural progenitor cells are a specialized type of stem cell specific to the nervous system that have the ability to transform into the various types of tissues damaged and destroyed by the MS disease process. Researchers at the Tisch Center have developed a way to get mesenchymal stem cells to differentiate into neural progenitor cells, and hope that by injecting these cells directly into the spinal fluid the NP cells will directly target the regenerative mechanisms of the central nervous system (click here). The stem cells themselves may act to repair damaged tissues, but they’ve also been shown to have the ability to recruit existing stem cells within the brain and spinal cord to jumpstart the body’s own repair mechanisms.
It’s important to remember that both of these studies represent a very different approach to stem cell therapy for MS than HSCT. The primary goal of HSCT is to reboot a patient’s immune system; HSCT does nothing to directly address the damage that has already been caused by the disease, but rather seeks to disrupt the disease process. Taking a different approach, the trials being conducted at the Cleveland Clinic and the Tisch MS Center seek to effect repairs on the damaged brains and spinal cords of MS patients, albeit through two different methodologies. HSCT and the reparative therapies being tested in the FDA trials have little in common other than the fact that they both use stem cells in an attempt to treat MS.
I’m sure that many patients reading this are aware that there are clinics in Central America, Asia, and Europe offering regenerative stem cell therapy to patients at hefty price tags. Some of these clinics aggressively market their services, and typically charge $20,000-$40,000 for a single round of stem cell therapy. Various Facebook pages, blogs, websites and posts on MS Internet forums extol the virtues of the treatments these clinics provide, often offering glowing testimonials from patients they have purportedly treated. Although I don’t want to disparage any patient relating their genuine experiences with these clinics, I’ve known several MSers that have traveled to a variety of these clinics and undergone stem cell treatments, and unfortunately none of them have experienced anything in the way of significant or lasting benefit.
I would caution anybody considering treatment in Panama, Costa Rica, Germany, India, or any of the other clinics offering stem cell therapy without any published scientific proof of the effectiveness of their treatments to think long and hard before committing substantial amounts of money for a therapy that, according to the experiences of people that I actually know, has very little chance of working. The two legitimate trials I outlined above both involve multiple treatments given over an extended period of time, using cells that have undergone lengthy (months long) processes of multiplication and/or differentiation in the laboratory before being transplanted back into the patient. Such regimens are not followed by the “pay to play” clinics; instead, they generally infuse stem cells back into the patient soon after they are harvested, and offer extremely limited, if any, follow-up care.
Additionally, some of these clinics don’t use a patient’s own stem cells for treatment, but rather umbilical cord cells, on which far less research has been done. The use of stem cells not derived from the patient themselves opens up all kinds of questions regarding safety and efficacy, as the cells are genetically different from the tissues they are meant to repair. If any of these clinics regularly achieved anything close to the number of successful outcomes that they claim, they would surely publish their results in legitimate scientific journals and reap the personal and professional accolades that would follow. Can you say Nobel Prize? Instead, they publish marketing materials and partner with travel agencies. Reason enough for skepticism. In short, let the buyer beware.
Stem cell therapy holds tremendous potential for the treatment of multiple sclerosis, and provides much reason for hope. The efficacy of HSCT for treating very aggressive relapsing remitting multiple sclerosis is well documented, and the safety of this treatment regimen has increased dramatically as practitioners have perfected the process. Regenerative stem cell therapy, of the type currently being trialed at the Cleveland Clinic and Tisch MS Research Center of New York, is still in its infancy, but is bursting with promise, possibly holding the key to repairing the damage done by multiple sclerosis and restoring function robbed by the disease. As with all new therapies, though, it is vitally important to not let hope eclipse reason, or let hype cloud judgment. We are at the dawn of a new age, and I fully believe that the use of stem cells will revolutionize the practice of medicine. Research into the use of stem cells to treat MS is quickly picking up steam, and in combination with other emerging therapies, rays of hope are finally being shone upon the disease and those afflicted with it. It’s about time, don’t you think?
Thursday, December 22, 2011
Bits and Pieces: Happy Holidays Edition
(note: for those who receive this via e-mail, this post contains some video links, which can only be accessed from the Wheelchair Kamikaze website itself)
Well, it's again that time of year when a jolly fat man in a red velvet suit slides down chimneys and performs home invasions in an endless search for cookies and milk. Yup, it appears my uncle Bart is once again off his meds. I think it may be time for an intervention…
The holiday season is upon us, but unlike in conflicts of old during which a truce was often called in reverence to the holiday, the war that MS wages upon its victims continues unrelentingly, as does the attempted counterattack being fought against MS. There's plenty of research news to catch up on, and I'll throw a few other seasonal tidbits on the Yuletide fire to keep with the holiday spirit.
So, grab a nice cup of spiked eggnog, hot cider, or kosher wine, as I submit the following items for your perusal, all the while wishing you a Merry Christmas, Happy Hanukkah, Good Kwanzaa, or, if none of those float your boat, then just a damn good week…
♦ Speaking of alcoholic beverages, a new study out of Belgium suggests that drinking booze and coffee, and eating fish may help delay disease progression in patients with relapsing multiple sclerosis (click here-may require free registration, which is well worth it for the wealth of information available on this site). Unfortunately, the same does not hold true for patients with progressive MS, whose intake of these substances didn't significantly alter the course of their disease. As usual, progressive patients are the redheaded stepchildren of the MS world, spanked in the ass and sent to bed without dinner. Smoking was found to be detrimental to both relapsing and progressive patients.
Researchers found that relapsing patients who drank moderate amounts of alcohol and coffee, and ate fish at least two times a week, had a significantly increased time of disease progression to the level of EDSS 6, which is defined as the point at which a patient needs intermittent or unilateral constant assistance (cane, crutch or brace) to walk 100 meters with or without resting. Interestingly, in progressive patients it appears that the type of fish eaten (fatty or lean) impacted the speed of progression. Patients who ate fatty fishes (such as salmon, tuna, and mackerel) fared worse than those eating leaner varieties.
The discrepancy between relapsing and progressive patients' response to the dietary influences studied is likely attributable to the fact that relapsing disease is more inflammatory than progressive, and alcohol, coffee, and fish all have anti-inflammatory properties. Fatty fishes tend to pick up more environmental toxins, which may account for their association with increased rates of disease progression.
♦ A major new study recently completed by the Mayo Clinic in collaboration with the Cleveland Clinic has the potential to completely rewrite the scientific understanding of how multiple sclerosis develops and progresses (click here, same deal with possibly having to register). For many years, the conventional wisdom has been that MS started deep in the brain, attacking white matter (myelin) first, and only later disrupting a patient's gray matter (nerve cells). In examining brain biopsies taken from patients very early in the disease process, the researchers discovered that, much to their surprise, there was inflammation and damage being done to the meninges (the membrane covering the brain), the subarachnoid space (which contain cerebrospinal fluid), and the gray matter, occurring concurrently with early damage done to myelin. In other words, instead of working from the inside out, the disease may work from the outside in.
The fact that such a basic understanding of the disease may have been wrong for many decades only underscores how pitifully little is actually known about the MS disease process, despite the tremendous amount of time spent researching the disease. This could be a discovery of terrific import, and may eventually turn the way MS is treated completely on its head. Interestingly, some news outlets reported this research as finding neurodegeneration in the absence of inflammation, which is incorrect. One of the lead researchers on this project, Dr. Claudia Lucchinetti, has done some previous research that hinted at such neurodegeneration, but this most recently released research finds inflammation throughout the CNS of patients very early in the disease process. Here's a video of Dr. Lucchinetti talking about the discovery:
♦ Another new hypothesis about the genesis of multiple sclerosis is being published in the Quarterly Review of Biology. Entitled "MS Is Not a Disease of the Immune System", the paper suggests that MS is a result of faulty lipid metabolism, or the inability of the body to properly uptake, breakdown, and release lipids (click here). In this scenario, an accumulation of toxic lipids, in conjunction with other genetic or environmental factors, leads to damage in the central nervous system and eventually to a clinical presentation of multiple sclerosis. Rather than get into a lengthy explanation here, I'll refer you to the blog of Nicola Griffith, a writer living with MS (click here). Her description of this new theory is quite clear and extremely well presented, and a big thanks to Nicola for bringing this research to my attention.
♦ In keeping with the Christmas spirit, here's a lovely little story about an MS patient who was ripped off to the tune of $65,000 by her health aide (click here). I'm constantly coming across news stories about MS patients being assaulted, having their wheelchairs or scooters stolen out from under them, or otherwise being criminally victimized. WTF? Some humans simply don't deserve to be called humans. Now, we've all committed deeds that would make our mothers embarrassed to have borne us, but by and large the MS patients I've met are a pretty decent lot. Who knows, maybe some were scumbags before getting sick, but I don't get that sense. Although my rational self knows not to expect some sort of easily discerned "just universe", it still galls me to no end to watch the nightly news and see a parade of murderers, rapists, and child molesters sauntering effortlessly past the cameras, even while handcuffed. Why do these evil, demented, living and breathing turd blossoms enjoy robust good health while so many decent folks I know suffer miserably with a progressively crippling disease? That's strictly a rhetorical question, no need for any direct answers…
♦ Here's a thought-provoking piece published in the Washington Post, entitled "Was My Doctor Loyal to Me, or to the Drug Companies?" (click here). Now, there's a question that has crossed the mind of many a patient. With the tentacles of Big Pharma reaching into almost every aspect of what I like to call the Medical Industrial Complex, one can never be sure if treatments are being offered because of their superior efficacy, or because the physicians prescribing them have been influenced by the largess of the drug companies producing them.
My personal neurologist doesn't allow any pharmaceutical reps into his clinic, and the facility is completely absent posters, pens, pads, or promotional materials of any type hyping one drug or another. This is in direct contrast to the offices of all of my other physicians, upon which the name of one pharmaceutical or medical device product or another is emblazoned on every available surface, placed strategically to be ingested wherever my gaze happens to land. Why the practice of Pharma companies paying off doctors (either as "consultants" who barely do any consulting, or with invitations to lavish "educational symposiums" held in luxury resorts, whose primary teaching function appears to be how to sink an 18 foot putt) is legal is beyond me. When I worked in the music industry, the practice of paying off radio station managers and DJs to play our labels' music was called "payola", and people who were caught engaging in this practice wound up doing time in the clink. Not so with Big Pharma and physicians, though. In an industry where the health and well-being of an entire nation is concerned, barely camouflaged bribery is considered business as usual. Ho Ho Ho, Merry Christmas…
♦ This is the time of year when charities rake in a tremendous amount of donations. I'm often asked which MS nonprofits are most worthy of my friend's and family's money, so here's a list of very worthy MS research organizations. These are all smaller outfits, dwarfed by the giant elephant of the MS nonprofit universe, the National Multiple Sclerosis Society. Unlike some other bloggers and activists, I have nothing against the NMSS. Although it might not always act quite the way I would like it to if I were king of the forest, the organization does do a tremendous amount of good across a wide array of issues impacting patients suffering from Multiple Sclerosis. The problem is that to the general public the NMSS is THE multiple sclerosis organization to which to donate, leaving the little guys scrambling for scraps. The following organizations all do exceptional work, and are very deserving of any donations that flow their way:
· The Accelerated Cure Project (click here)-this group maintains a large MS repository, consisting of blood samples and extensive demographic data taken from well over 1000 MS patients, making it an extremely valuable resource to researchers worldwide. 2011 saw the 50th research project done using repository samples and data, and the ACP is currently putting together a repository devoted to neuromyelitis optica and other demyelinating diseases.
· The Myelin Repair Foundation (click here)-the MRF is devoted to revolutionizing the way MS research is done, dramatically shortening the time it takes to shepherd a potential treatment from discovery through regulatory approval. The MRF, founded by Scott Walker, himself an MS sufferer, is especially devoted to hastening the development of neuroregenerative and neuroprotective therapies, which are the holy grail of MS research.
· The Multiple Sclerosis Research Center of New York (click here)-this is the research arm of the MS clinic that takes care of me, under the direction of Dr. Saud Sadiq. This completely independent research center is on the cutting edge of MS research, and recently won approval for one of the first regenerative MS stem cell trials to be done in the United States. In addition to extensive stem cell research, MSRCNY scientists are hard at work exploring new and more effective MS treatments, looking for biomarkers to increase the diagnostic accuracy of the disease, and providing dramatic symptomatic relief for MS sufferers. Lots of outside of the box thinking going on in this place.
· The Buffalo Neuroimaging Analysis Center (click here)-BNAC is at the forefront of CCSVI research, conducting extensive imaging studies on MS and non-MS subjects that have already shed much light on the CCSVI hypothesis and its role in the fight against MS. Led by world-class researcher Dr. Robert Zivadinov, BNAC is also undertaking one of the first CCSVI treatment trials being done to strict scientific standards. CCSVI has the potential to profoundly change our understanding of MS, and BNAC has the potential to be the organization that unlocks the secrets of CCSVI.
· The CCSVI Alliance (click here)- the CCSVI Alliance promotes education and research about CCSVI and its relationship to Multiple Sclerosis by providing objective information to the MS community, supporting medical investigations of CCSVI, and fostering collaboration among patients, advocates, and professionals. They are currently working on patient education programs at major conferences around the country, and are working hard to encourage the interdisciplinary cooperation needed to unravel the mystery of CCSVI.
Well, as we come to the end of this post, I realize it might not have been filled with as much joviality as I initially intended. Sorry for that, and despite the "Bah Humbug" nature of much of the above, I'd like to sincerely wish all WK readers (and all non-WK readers) a scintillating holiday season. May all your wishes be realized, and all your dreams come true.
Here's a holiday song that seems especially fitting given the current state of world economic turmoil, which finds far too many struggling just to keep their heads above water. It's performed by a great rock 'n roll band that has long been vastly underrated. As the song says, "Have yourself a Very Merry Christmas, Have yourself a good time, But remember the kids who got nothing, As you're drinking down your wine".
Amen
RIP Christopher Hitchens
Related articles
Tuesday, August 30, 2011
A Bit, a Piece, and Some Photos…
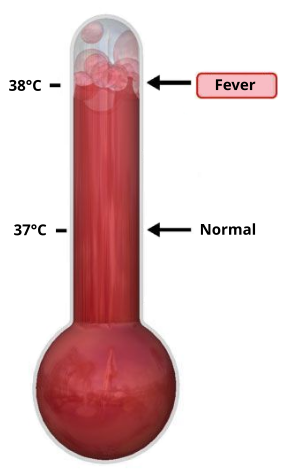
Image via Wikipedia
Sorry about the nearly two-week gap since my last post, but in addition to earthquakes, hurricanes, and my birthday, I've also been dealing with a persistent low-grade fever that seems to be related to the monthly IVIG infusions I've been receiving. My doctors claim that IVIG doesn't cause such long-term fevers, but the fevers do seem to have started when I started IVIG, and have increased in frequency in the five months that I've been on the stuff. As I'm sure most of you know, those of us with damage to our central nervous systems are especially sensitive to increased body temperatures, so these fevers have been draining both mentally and physically.
The real kick in the ass is that IVIG is the first treatment I've been on that has actually shown any benefit, having restored some strength to my extremely weak right side. Frustratingly, it doesn't seem to have stopped the progressing weakness on my left side, which is quite distressing since I use my relatively strong left side to make up for my extremely gimpy right side. Once the left side goes, I'm all out of sides, and thus shit out of luck, since I'm a mere Earthling and don't have the benefit of the additional extremities sported by some of our extraterrestrial friends. Damn, if only I was one of those six armed, six legged bastards from the planet Mu, but then again if I were I'd be forced to exist on a diet made up exclusively of tremendous brussels sprouts, and I absolutely detest brussels sprouts. Karen adores them, though, and it's only due to the magical power of love that our relationship has survived this calamitous obstacle.
Anyway, enough about me. I thought I'd share a couple of interesting MS related news items, and a new batch of photos that I've added to the Wheelchair Kamikaze photo gallery, which can be found on the left side of this blog. It's been a while since I've added any photos, so it's about time. I'll have a bunch more to post sometime soon, so stop needling me, see, stop riding me, or I'll have to reach through the Internet and sock you right on the kisser. Yikes, suddenly I'm writing like a 1930s movie gangster, so before any more such silliness ensues, let's get on with it…
· The first FDA approved stem cell trial involving multiple sclerosis patients is now underway at the Cleveland Clinic in Cleveland, Ohio (click here). The trial is using mesenchymal stem cells, harvested from a patient's own bone marrow, and then infused intravenously back into the patient after the cells have been cultivated and multiplied in a carefully controlled lab environment. Although this is a very small phase 1 trial, at least one of the initial patients is already reporting some improvements. The mesenchymal stem cells (MSC's) are thought to work by both regulating the body's immune response and initiating the repair of damaged nerve tissues, and offer the extremely exciting prospect of not only stopping the disease but also reversing some of the damage it does to the nervous system. Another small trial, conducted by Dr. Neil Scolding in England, reported encouraging results, finding that patients treated with intravenously infused MSC's showed stability and even some improvement in symptoms after one year (click here). Much larger stem cell trials are currently being readied throughout Europe, and are slated to get going later in 2011 (click here). Similar stem cell treatments are being offered in various overseas clinics at extremely high prices, but the outcome reports from patients who have traveled for these treatments have been mixed
· Yet more idiocy involving the use of medical marijuana to treat MS in the United States has once again made the news, this time in New Jersey, where an MS patient has been tried, convicted, and sentenced to five years in prison for growing 17 marijuana plants for his own use (click here). During his trial, the judge determined that the fact that the defendant has MS and was harvesting the plants as a form of medicine not be allowed to be told to the jury. The convicted man is now appealing the case to the State Supreme Court, and had been out on bail, but as of a few days ago is in prison beginning to serve his sentence. The kicker is that New Jersey now has a law allowing medical marijuana to be used for certain conditions, including MS, but it was passed after this man was first arrested. As I've written about previously (click here), the draconian anti-marijuana laws here in the US - enacted in the mid-20th century largely to protect the cotton industry from the growers of hemp - don't even allow for the testing of pharmaceutical products derived from marijuana, such as Sativex, an anti-spasticity spray available throughout Canada and much of Europe. Sheesh…
Okay, now for the photos I know you've all been waiting for with bated breath. These were all taken between within the last 12 months, with a camera mounted on the arm of my wheelchair. Most were shot in either Central Park or Hudson River Park. I hope you like them. Please click on the thumbnails below to view a larger image. I'd love to know if you have any favorites, or even if you happen to despise any of them, so please feel free to leave some comments…
 |  |  |  |
 |  |  |  |
 |  |  |  |
 |  |  |  |





